-
Epoxidation of cyclic allylic alcohols on titania-silica aerogels studied by attenuated total reflection infrared and modulation spectroscopy
A. Gisler, T. Bürgi and A. Baiker
Journal of Catalysis, 222 (2) (2004), p461-469


DOI:10.1016/j.jcat.2003.12.009 | unige:14772 | Article HTML | Article PDF
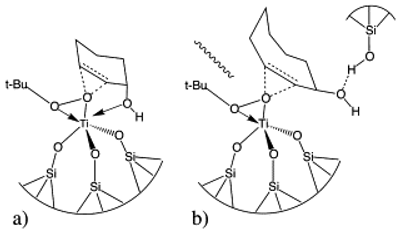
Epoxidation of cyclohex-2-en-1-ol and cyclooct-2-en-1-ol on titania–silica aerogel catalysts using t-butylhydroperoxide (TBHP) as oxidant was studied by in situ attenuated total reflection (ATR) Fourier transform infrared spectroscopy. Probing of the catalytic liquid–solid interface revealed different adsorption behaviors for the two allylic alcohols on the aerogel. Cyclohexenol was found to adsorb stronger and less reversible on the catalyst surface and Ti sites than cyclooctenol. The spectroscopic measurements under working conditions support the previously proposed hydroxy-assisted mechanism for the formation of cyclohexenol oxide and the silanol-assisted mechanism for cyclooctenol epoxidation. The evidence of the former is traced to the occurrence of a framework vibration upon adsorption of cyclohexenol, whereas the latter is supported by large negative bands of the silanol groups at 3700 and 980 cm−1 in the case of cyclooctenol epoxidation.